On the Road without mineral Oil
“Electric cars!” — this is what most people reply when asked how we will drive in the post-fossil age. Products like Elon Musk’s Tesla seem to affirm this notion. But battery-powered cars have a number of critical drawbacks.
A little excercise, which teachers of physics may set their students, shows the problem. The question: Which power (heat content per second) is put out by a fuel station pump? These pumps deliver around 35 l/min and a liter of gasoline contains 30 MJ: Therefore, the fuel flow packs 17.5 megawatts!! Even if only one car pulls up per hour (a gross understatement for busy gas stations) to have its tank completely filled up (80 l), the station must deliver a time-averaged power of 0.7 MW, similar to a small electric locomotive.
It’s hardly surprising that recharging an electric car’s accumulator takes much longer than fueling up a gasoline or diesel car and that the electric vehicle has a massively shorter range.
Additionally, batteries have to contain both reaction partners needed to release energy, while combustion motors one of the two (oxygen) is taken from the air. For this reason, accumulators (most of all the lithium-ion type) pose a substantial risk of explosion.
Production and decomissioning of batteries are complex from an environmental perspective, as toxic and corrosive substances need to be handled.
Are there alternatives to the electric car when it comes to mineral-oil-free mobility? Luckily: yes! The DFR can help here.
Hydrogen
Popularized by such stunts as Arnold Schwarzenegger rolling through Los Angeles in a H2-SUV, the lightest element is considered the fuel of the future by many. From the oceans it can be produced via water splitting in near infinite amounts, reaction product is water again: a closed cycle. What is needed is a powerful energy source: Separating oxygen and hydrogen is an endothermic process — the energy released during the combustion must be put in. Hydrogen is an energy carrier, not an energy source, as on earth it hardly appears in isolated form.
The DFR is an excellent energy source to produce hydrogen — through the HOT ELLY-Process (electric-thermal) or the sulphur-iodine-cycle (thermal at 830 °C), production from water becomes cheaper than natural gas splitting, which is the common method today.
Hydrogen as a vehicle fuel has some problems, though: most of all, it is highly volatile! The minuscule H2 molecules diffuse through nearly any metal. Modern materials may counteract this effect, nonetheless large pressurized or cryogenic tanks are needed. Compression and/or liquefaction absorb nearly as much energy as the production of the hydrogen itself. For this reason, industrial application may make more sense, e.g. utilization for the reduction of iron ore instead of coke. To fill up vehicle tanks, one should rather have a substance which stays liquid or nearly liquid under typical conditions on earth’s surface: 20 °C, 1 bar. Using additional production steps, hydrogen can be used to liquefy other elements: This is called XtL-fuels — substance X-to-Liquid.
Coal-to-Liquid: synthetic Gasoline
DFR process heat turns coal into gasoline or diesel via Fischer-Tropsch-Process. Until now, fuel created this way was far more expensive than mineral oil products, but ultra-cheap energy from the DFR makes all the difference.
If coal is not wanted to avoid carbon dioxide emissions or because it is not available, the syngas, a mixture of carbon monoxide and hydrogen that appears as an intermediate product during Fischer-Tropsch-Synthesis, can also be created by plasma recycling of waste containing hydrocarbons: Domestic and industrial waste, leftovers from agriculture and forestry, sewage sludge etc. In a plasma converter the chopped up waste is heated by electric arcs to several 1000 °C, causing it to decompose into its elemental constituents. At the bottom of the converter, metal and silicate slag deposits — raw materials for re-use in the industry —, at the top syngas escapes.
Electricity for the arc is plentiful next to a DFR, and the high process temperature can give the waste mixture an extra boost via a hot gas jet. The syngas is further processed through Fischer-Tropsch into liquid hydrocarbons.
DFR energy turns the plasma converter into an economical alternative to toxic waste repositories such as Herfe-Neurode, as it is able to destroy (halogenous-)organic poisons.
Nitrogen-to-Liquid: Ammonia and Hydrazine
From hydrogen and aerial nitrogen, ammonia (NH3) can be manufactured via Haber-Bosch-Process using DFR process heat. This gas is suitable for powering piston and turbine engines, needing — contrary to hydrogen — only mildly pressurized tanks (10 bar). Another possibility is further processing into hydrazine.

Hydrazine (N₂H₄) is well-known from spaceflight technology. But it is more than just a rocket fuel. In piston or turbine engines, it burns as well as gasoline or kerosine, with some minor adjustments of injection and ignition points, similar to the adaptions for LPG. Its heat value is about half of that of hydrocarbons, but this can be compensated by an admixture of water — the resulting steam will increase the pressure difference moving the pistons or turbine blades.
DFR process heat enables its economical production from hydrogen and aerial nitrogen; burning it produces nitrogen and water steam. The substance in itself is toxic, but if released into the environment, it decomposes quickly, while mineral oil products linger for a long time, burdening the environment. Filling up occurs through an adapter similar to those for LPG. Huge oil tankers and pipelines will soon be a thing of the past — when next to each city a small nuclear process heat plant springs up, creating hydrazine for local customers!
Apart from refittung classical internal combustion engine, another, more efficient type of utilization is possible: Hydrazine fuel cells. While these do create electricity for drive motors, they have little in common with batteries. In fuel cells, a chemical combustion takes place, but the electron transport way from reductor to oxidizer is increased to a macroscopic distance using membranes, building up voltage that powers a current. The best-known type of fuel cell uses hydrogen. These are very expensive, though, as aggressive acids are formed within, necessitating the coating of the electrodes with platinum. Hydrazine cells, on the other hand, contain an alkaline liquid which is far less reaction-prone, so that nickel or cobalt electrodes suffice. The purchase of a hydrazine fuel cell car is about as expensive as that of a mid-range diesel. Also, the efficiency is about 2.5 times greater than a piston engine’s, meaning that a halved heat value results in 25% more range per full tank. If the hydrazine is produced using nuclear energy, the range per monetary unit is even doubled.
Ammonia fuel cells have been tested, too.
This technology gives us an inexpensive car with great driving dynamics and low fuel costs, without any harmful exhaust fumes, risky gas tanks or massive, explosion-prone accumulators: All strengths of the electric car — none of its weaknesses!
Fans of powerful muscle cars may, of course, look at this sceptically, as there is no resounding engine roar, only the quiet purr of electric motors. But these people need not be left standing: Their cars can easily be adapted to the burning of hydrazine or ammonia. The thunder of the “V8 Interceptor” retains its impressiveness even when the exhaust tubes exhale nitrogen and water vapor.
Silicon-to-Liquid: Silane
From Europe to America in thirty minutes — on a hot tail of sand?! A little-known fuel option suitable for hypersonic aircraft with ram- or scramjets are silanes, silicon homologues of alcanes. They burn at 1400 °C, reacting with oxygen as well as aerial nitrogen to water and silicon nitrite, thus using 99% of air! Silicon nitrite is, notabene, a solid substance (sand): Only engines without moving components are suitable. Turbine blades don’t like solid particles slamming into them constantly, but the ram- and scramjets of futuristic Super-Concordes (beyond Mach 5!) will not have any blades. Modified Wankel engines could also be adapted to Silanes.
Boron
A truly exotic concept, deviating very far from common notions of fuel, is the semi-metal boron, which is usually mined from dry salt lake beds — extraction from seawater is also possible.
Boron is chemically inert in air, in pure oxygen it will burn with higher calorific value than all other chemical fuels that have been suggested except hydrogen which surpasses it with respect to energy per mass:
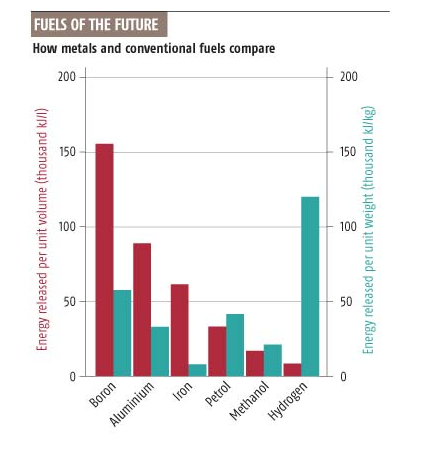
Heizwerte pro Masse und Volumen für Bor, Eisen und die wichtigsten herkömmlichen Treibstoffe. (Source)
Boron oxide is a rubber-like, doughy mass: Even more problematic for moving motor parts than silicate dust! The most practical method to use boron for vehicle propulsion could therefore be external combustion, having it react in a combustion chamber with pure oxygen which has been extracted from air by a pre-stage, heating a working medium for a turbine or a Stirling motor. The oxide is captures behind the combustion chamber. That way, “filling up” gets an unusual new meaning: The driver hands the full boron oxide container to the clerk of a supermarket or kiosk (keep in mind that boron doesn’t react with air and can therefore be sold in retail stores without additional safety measures) and gets a fresh load of boron dust for another 1000 km. The oxide is transported to a DFR plant, where it gets reduced by electricity or heat — thus, only one tank filling per car needs to be extracted!
This option is probably furthest from realization concerning motor technology and infrastructure — though the notion of a closed loop for chemical fuels is fascinating.
(See also: Tom Blees: “Prescription for the Planet”, chapter 5)
